This is an informational page for patients to learn facts about a specific recall and find out if an implant that was packaged in nonconforming packaging was used during your procedure. Click the links below to see if your implant is affected by a recall.
Exactech Recall Information
2024
Important Patient Information Regarding Exactech Polyethylene Patellas
This is an informational notice about Exactech polyethylene patellas that were packaged in nonconforming bags.
We are conducting a medical device recall for nonconforming packaging in order to retrieve unused inventory. If a patient has a patella component within the scope of this recall, this does not mean that the patella component needs to be replaced.
If you are experiencing any new or worsening knee swelling, pain while walking, inability to bear weight, grinding or other noise, instability, or any new symptoms of clicking in your knee, you should contact your health care provider for further investigation of the cause of the new or worsening symptoms. These could include implant factors as well as patient factors (age, sex, physical characteristics, activity level, etc.), surgical factors (technique, alignment, etc.), and postoperative rehabilitative factors. Normal patient monitoring and follow-up per your surgeon’s standard of care is recommended. Revisions of well performing patellas from nonconforming packaging are not recommended.
You can look up your information here to learn if an implant that was packaged in nonconforming packaging was used during your procedure.
The following products are affected:
Scroll right to view the full table on mobile devices.
| Catalogue Number | Product Name | ARTG Number |
|---|---|---|
| 200-02-26, 200-02-29, 200-02-32, 200-02-35, 200-02-38, 200-02-41 | Optetrak Three Peg Patella 26MM – 41MM | 279041 |
Learn about your implant
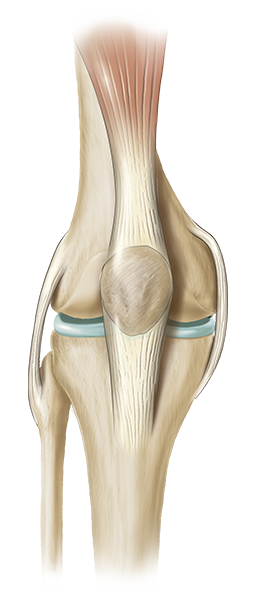
The knee is the largest joint in the body and is made up of the lower end of the thigh bone (femur), the upper end of the shin bone (tibia), and the knee cap (patella).
Important Patient Information Regarding the Equinoxe Shoulder System
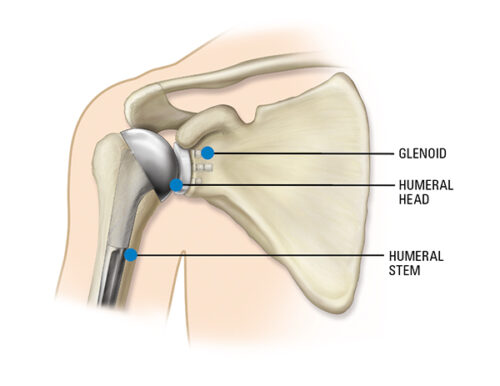
This is an informational notice about Equinoxe Shoulder polyethylene humeral liners and anatomic glenoids that were packaged in nonconforming bags.
We are conducting a medical device recall for nonconforming packaging in order to retrieve unused inventory. If a patient has a shoulder component within the scope of this recall, this does not mean that the shoulder component needs to be replaced.
If you are experiencing any new or worsening pain or swelling, inability to use your arm, grinding or other noise, or weakness around your implanted device, you should contact your health care provider for further investigation of the cause of the new or worsening symptoms, which could include implant factors as well as patient factors (age, sex, physical characteristics, activity level, etc.), surgical factors (technique, alignment, etc.), and postoperative rehabilitative factors. Normal patient monitoring and follow-up per your surgeon’s standard of care is recommended. Revisions of well performing shoulders from nonconforming packaging are not recommended.
If you would like to read the full FDA communication, <click here>. An excerpt is below:
“Recommendations for Patients:
-
- If your Equinoxe Shoulder System is functioning well and you have no pain or symptoms, the FDA does not recommend surgery to remove a well-functioning device.
- Contact your health care provider if you have an Equinoxe Shoulder System implanted and you are experiencing any new or worsening pain or swelling, inability to use your arm, grinding or other noise, or weakness around your implanted device.”
You can look up your information here to learn if an implant that was packaged in nonconforming packaging was used during your procedure. Please use the following link to see if your shoulder liner is affected by this recall (you will need your serial number):
The following products are affected:
Scroll right to view the full table on mobile devices.
| Product Number | Product Description | ARTG |
|---|---|---|
| 314-01-0X | EQUINOXE GLENOID, KEELED ALPHA | 159609 |
| 314-01-1X | EQUINOXE GLENOID, KEELED BETA | 159609 |
| 314-02-0X | EQUINOXE GLENOID, PEGGED ALPHA | 264381 |
| 314-02-1X | EQUINOXE GLENOID, PEGGED BETA | 264381 |
| 314-02-2X | EQUINOXE POST AUG GLENOID, LEFT | 278648 |
| 314-02-3X | EQUINOXE POST AUG GLENOID, RIGHT | 278648 |
| 314-13-0X | EQUINOXE CAGE GLENOID, ALPHA | 270232 |
| 314-13-1X | EQUINOXE CAGE GLENOID, BETA | 270232 |
| 314-13-2X | EQUINOXE CAGE GLENOID, POST AUG, LEFT | 293762 |
| 314-13-3X | EQUINOXE CAGE GLENOID, POST AUG, RIGHT | 293762 |
| 320-36-0X 320-38-0X 320-42-0X 320-46-0X | EQUINOXE 145-DEG PE 36 – 46MM HUM LINER | 264172 |
| 320-36-1X 320-38-1X 320-42-1X 320-46-1X | EQUINOXE 145-DEG PE 36 – 46MM CONST HUM LINER | 279721 |
More about the Equinoxe® Shoulder System:
The platform system allows conversion of a primary or fracture shoulder replacement to a reverse without the need to remove the already well-fixed stem. The high-quality implants are designed to:
- Help match each individual patient’s bone structure
- Preserve a patient’s natural anatomy
- Work in a variety of procedures
- Address unique clinical challenges
To learn more about your implant, click here
Previous
Knee and Ankle Polyethylene
This is to inform you of recent observations made by Exactech regarding the clinical performance of our Knee and Ankle polyethylene inserts.
The following products are affected:
Scroll right to view the full table on mobile devices.
| Product Number | Product Description | ARTG |
|---|---|---|
| Knee Recall | ||
| 208-2X-XX | Optetrak Tibial Insert, CC w/ Retaining Screw | 277743 |
| 264-2X-XX | Optetrak RBK PS, Hi-Flex Tibial Insert | 277745 |
| 200-2X-XX | Optetrak Tibial Insert, CR Slope Neutral, size 1-5 | 278343 |
| 200-5X-XX | Optetrak Tibial Insert, CR Slope Neutral, size 0 | 278343 |
| 200-6X-XX | Optetrak Tibial Insert, CR Slope +, size 1-5 | 278343 |
| 200-7X-XX | Optetrak Tibial Insert, CR Slope ++, size 1-5 | 278343 |
| 02-012-51-XXXX | Optetrak Logic Tibial Insert, CRC | 284907 |
| 02-012-47-XXXX | Optetrak Logic Tibial Insert, CR Neutral | 285442 |
| 02-012-48-XXXX | Optetrak Logic Tibial Insert, CR Slope + | 285442 |
| 02-012-49-XXXX | Optetrak Logic Tibial Insert, CR Slope ++ | 285442 |
| 02-012-35-XXXX | Optetrak Logic Tibial Insert, PS | 285502 |
| 02-012-44-XXXX | Optetrak Logic Tibial Insert, PSC | 285503 |
| 204-2X-XX | Optetrak Tibial Insert, PS | 285677 |
| 02-012-38-XXXX | Optetrak Logic RBK Tibial Insert, Posterior Stabilized | 285911 |
| 244-2X-XX | Optetrak Tibial Insert, Hi-Flex, PS | 286119 |
| Ankle Recall | ||
| 350-41-XX/350-42-XX | Mobile-bearing total ankle prosthesis | 308700 |
Exactech Knee & Ankle Polyethylene Patients: What to do
Please use the following link to see if your hip liner is affected by this recall (you will need your serial number):
Connexion GXL & Conventional Hip Liners
This is to inform you of recent observations made by Exactech regarding the clinical performance of Connexion GXL and conventional polyethylene acetabular liners sold in the United States. The product-specific information is listed in the links below.
The following products are affected:
Scroll right to view the full table on mobile devices.
| Product Number | Product Description | ARTG |
|---|---|---|
| 130-XX-XX | GXL NUETRAL LINER | 287411 |
| 132-XX-XX | NV GXL LNR, LIPPED | 287411 |
| 136-XX-XX | NV GXL LNR, +5LAT | 287411 |
| 138-XX-XX | NV GXL LNR, 10 DEG FACE CHANGING | 287411 |
Exactech Hip Polyethylene Patients: What to do
Please use the following link to see if your hip liner is affected by this recall (you will need your serial number):