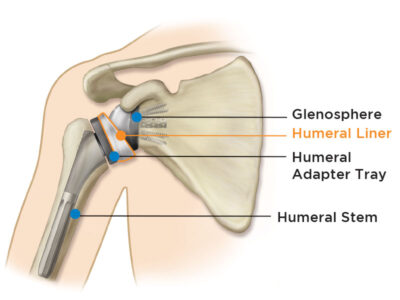
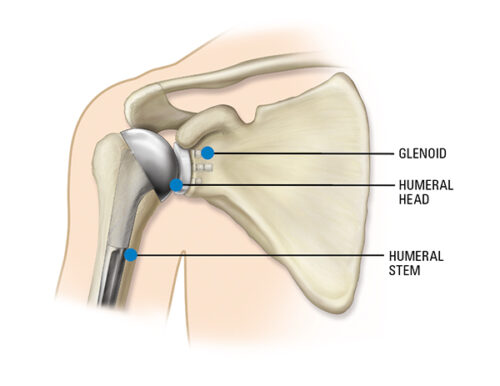
Important Patient Information Regarding the Equinoxe Shoulder System
This is an informational notice about Equinoxe Shoulder polyethylene humeral liners and anatomic glenoids that were packaged in nonconforming bags.
We are conducting a medical device recall for nonconforming packaging in order to retrieve unused inventory. If a patient has a shoulder component within the scope of this recall, this does not mean that the shoulder component needs to be replaced.
If you are experiencing any new or worsening pain or swelling, inability to use your arm, grinding or other noise, or weakness around your implanted device, you should contact your health care provider for further investigation of the cause of the new or worsening symptoms, which could include implant factors as well as patient factors (age, sex, physical characteristics, activity level, etc.), surgical factors (technique, alignment, etc.), and postoperative rehabilitative factors. Normal patient monitoring and follow-up per your surgeon’s standard of care is recommended. Revisions of well performing shoulders from nonconforming packaging are not recommended.
If you would like to read the full FDA communication, <click here>. An excerpt is below:
“Recommendations for Patients:
-
- If your Equinoxe Shoulder System is functioning well and you have no pain or symptoms, the FDA does not recommend surgery to remove a well-functioning device.
- Contact your health care provider if you have an Equinoxe Shoulder System implanted and you are experiencing any new or worsening pain or swelling, inability to use your arm, grinding or other noise, or weakness around your implanted device.”
You can look up your information here to learn if an implant that was packaged in nonconforming packaging was used during your procedure. Please use the following link to see if your shoulder liner is affected by this recall (you will need your serial number):
The following products are affected:
Scroll right to view the full table on mobile devices.
Product Number
| Product Description
| ARTG
|
| 314-01-0X | EQUINOXE GLENOID, KEELED ALPHA | 159609 |
| 314-01-1X | EQUINOXE GLENOID, KEELED BETA | 159609 |
| 314-02-0X | EQUINOXE GLENOID, PEGGED ALPHA | 264381 |
| 314-02-1X | EQUINOXE GLENOID, PEGGED BETA | 264381 |
| 314-02-2X | EQUINOXE POST AUG GLENOID, LEFT | 278648 |
| 314-02-3X | EQUINOXE POST AUG GLENOID, RIGHT | 278648 |
| 314-13-0X | EQUINOXE CAGE GLENOID, ALPHA | 270232 |
| 314-13-1X | EQUINOXE CAGE GLENOID, BETA | 270232 |
| 314-13-2X | EQUINOXE CAGE GLENOID, POST AUG, LEFT | 293762 |
| 314-13-3X | EQUINOXE CAGE GLENOID, POST AUG, RIGHT | 293762 |
320-36-0X
320-38-0X
320-42-0X
320-46-0X | EQUINOXE 145-DEG PE 36 – 46MM HUM LINER | 264172 |
320-36-1X
320-38-1X
320-42-1X
320-46-1X | EQUINOXE 145-DEG PE 36 – 46MM CONST HUM LINER | 279721 |
More about the Equinoxe® Shoulder System:
The platform system allows conversion of a primary or fracture shoulder replacement to a reverse without the need to remove the already well-fixed stem. The high-quality implants are designed to:
- Help match each individual patient’s bone structure
- Preserve a patient’s natural anatomy
- Work in a variety of procedures
- Address unique clinical challenges